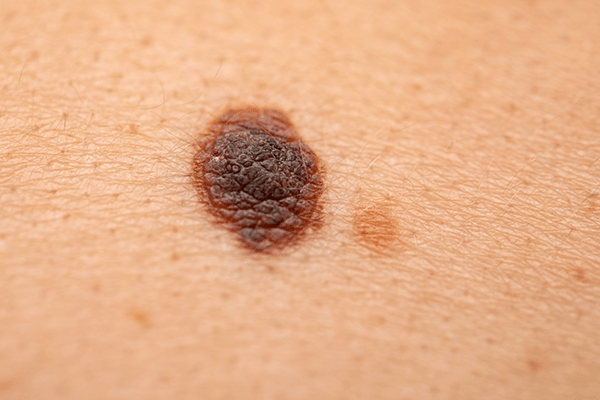
The recent FDA accelerated approval of lifileucel, known commercially as Amtagvi, marks a groundbreaking development in cancer therapy. This treatment is the first of its kind, a tumor-derived autologous T cell immunotherapy, specifically targeting patients with unresectable or metastatic melanoma who have undergone prior treatments. This advancement is a significant leap in precision medicine, opening new avenues for cancer treatment.
Understanding Lifileucel Lifileucel, developed by Iovance Biotherapeutics, is a form of cellular therapy incorporating tumor-infiltrating lymphocytes (TILs). This novel approach aims to utilize the patient’s own immune system to identify and eliminate melanoma cells, representing a major step forward in melanoma treatment.
Evidence from Clinical Trials The decision to approve lifileucel was influenced by the results of a comprehensive phase II trial conducted globally. This study observed substantial and enduring responses in patients treated with lifileucel. Notably, out of the participants, 23 showed an objective response, with three complete and 20 partial responses. These findings highlight a significant and lasting effect of the treatment, emphasizing its potential impact in cancer care.
Mechanism of Lifileucel Treatment The treatment with lifileucel involves an initial phase of lymphodepletion using cyclophosphamide and fludarabine. Post this regimen, patients receive IL-2 aldesleukin to aid in the expansion of the infused cells. The median dosage of lifileucel administered demonstrated an overall response rate of 31.5%, an encouraging sign for this therapy type.
Potential Side Effects and Warnings As with any medical treatment, lifileucel has its share of possible adverse reactions. Common side effects include fever, chills, fatigue, tachycardia, and diarrhea. Importantly, lifileucel carries a boxed warning highlighting serious potential risks such as treatment-related mortality, severe and prolonged cytopenia, infections, cardiopulmonary complications, and renal issues. Awareness of these risks is critical for both patients and healthcare providers.
The Road Ahead While additional studies are needed to further establish its clinical benefits, the FDA’s nod to lifileucel is a beacon of hope for those battling advanced forms of skin cancer. It’s anticipated that lifileucel will pave the way for more TIL-based therapies, extending benefits to patients with various solid tumors. The approval of lifileucel signifies a pivotal moment in the evolution of cancer treatment.
About Clear Health Pass™
Clear Health Pass™ is a bioinformatics, bio-surveillance, and health diagnostic platform for humans and Pets. Clear Health Pass™ is a minority/veteran-operated organization in partnership as tribal is a portfolio partner of The Native American Venture Fund (NAVF). Clear Health Pass Holdings, LLC, DBA Clear Health Pass™ is an appointed “Tribal Agent” for The Blue Lake Rancheria Economic Development Corporation (BLREDC), a federal, Section 17 Tribal Corporation, whose tribal sovereignty’s authority is derived from The Indian Reorganization Act Of 1934 (IRA), 25 U.S.C. § 477.
If you would like to know more information and investment opportunities with Clear Health Pass™ you can request information via this link

0 Comments